Data Management Planning
Research data management is a concept used to describe the managing, sharing, and archiving of research data to make it more accessible to the broader research community. Research data management provides an opportunity for a researcher to create a plan that will ensure that their data will be organized so that it can be shared with other researchers and archived for long term preservation.
Visit our data management planning webpages to learn more about the DMPs and the DMPTool.
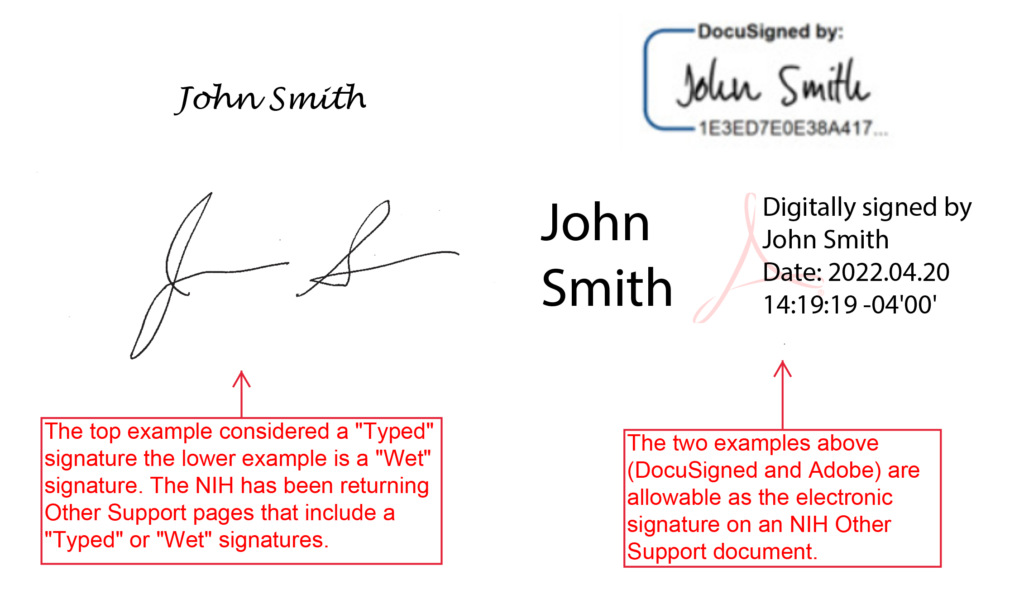
The NIH requires the Other Support document to be electronically signed.
“Wet” or “Typed” signatures are NOT ALLOWED.
If you are unfamiliar with the process of Creating and Applying a Digital Signature Certificate within Adobe, you may find this YouTube Video a helpful starting point.
Do not check the box “Lock document after signing”, when applying your Digital Signature Certificate.
If you do “Lock document after signing”, ORSP will NOT be able to combine your Other Support page with the rest of the documents being submitted to the NIH.
The NIH Other Support instructions states …
*Signature: Each PD/PI or other senior key personnel must electronically sign their respective Other Support form prior to submission. This signature certifies that the statements are true, complete and accurate.
The NIH has published clarifying information within the General Info section of the Frequently Asked Questions (FAQs) for Other Support.
Can Other Support be signed with a wet signature or a typed name?
No, wet and typed names will not be accepted as signatures. Electronic signatures are required, see related question below.
NIH does not allow electronic signatures on attachments in electronic applications. Will electronic signatures be accepted on Other Support submissions?
Other Support submissions must be submitted as a flattened PDF, after all signatures are obtained. Recipients and applicants may use the electronic signature software of their choice, and in alignment with their institutional practices. A typed name is not an electronic signature and is not acceptable.
Applicants and recipients must maintain supporting documentation to reasonably authenticate that the appropriate individual signed the form. Recipients must and make the documentation available upon request in accordance with 45 CFR Part 75.364.
Letter of Intent Sample/Template
The following Word documents are provided for reference only. ORSP must prepare and endorse any agreement with an outside entity related to sponsored activity.
Standard Marquette Agreements
The following Word documents are provided for reference only. Contact ORSP for assistance: ORSP must prepare and endorse any agreement with an outside entity related to sponsored activity.
Forms

